If the cells you would like to access are currently listed as unavailable or
you would like information on local partners in USA, Australia, New Zealand or Japan who can support order and delivery,
please get in touch via
Contact@EBiSC.org.
PFIZi032-A
B214c8
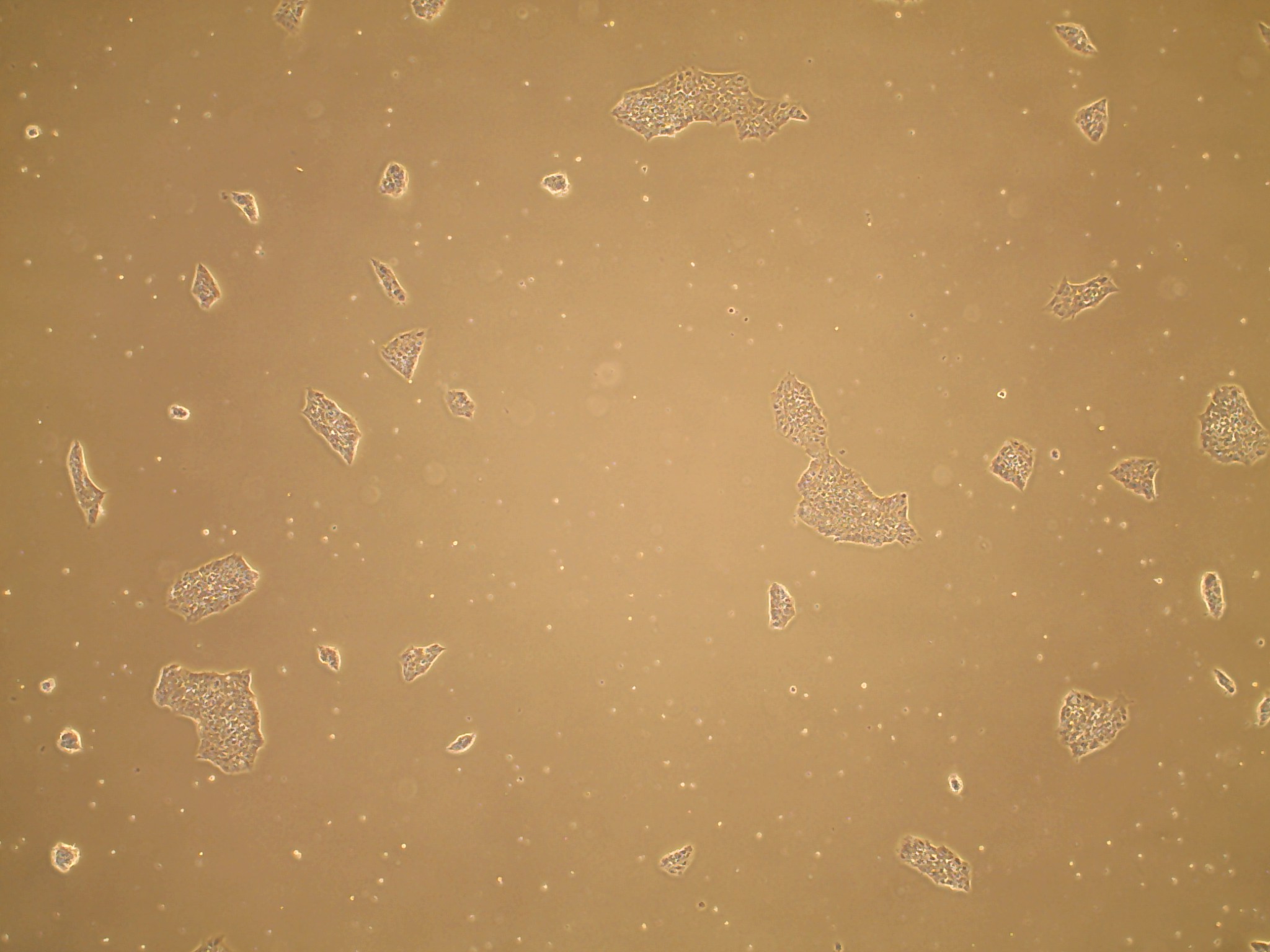
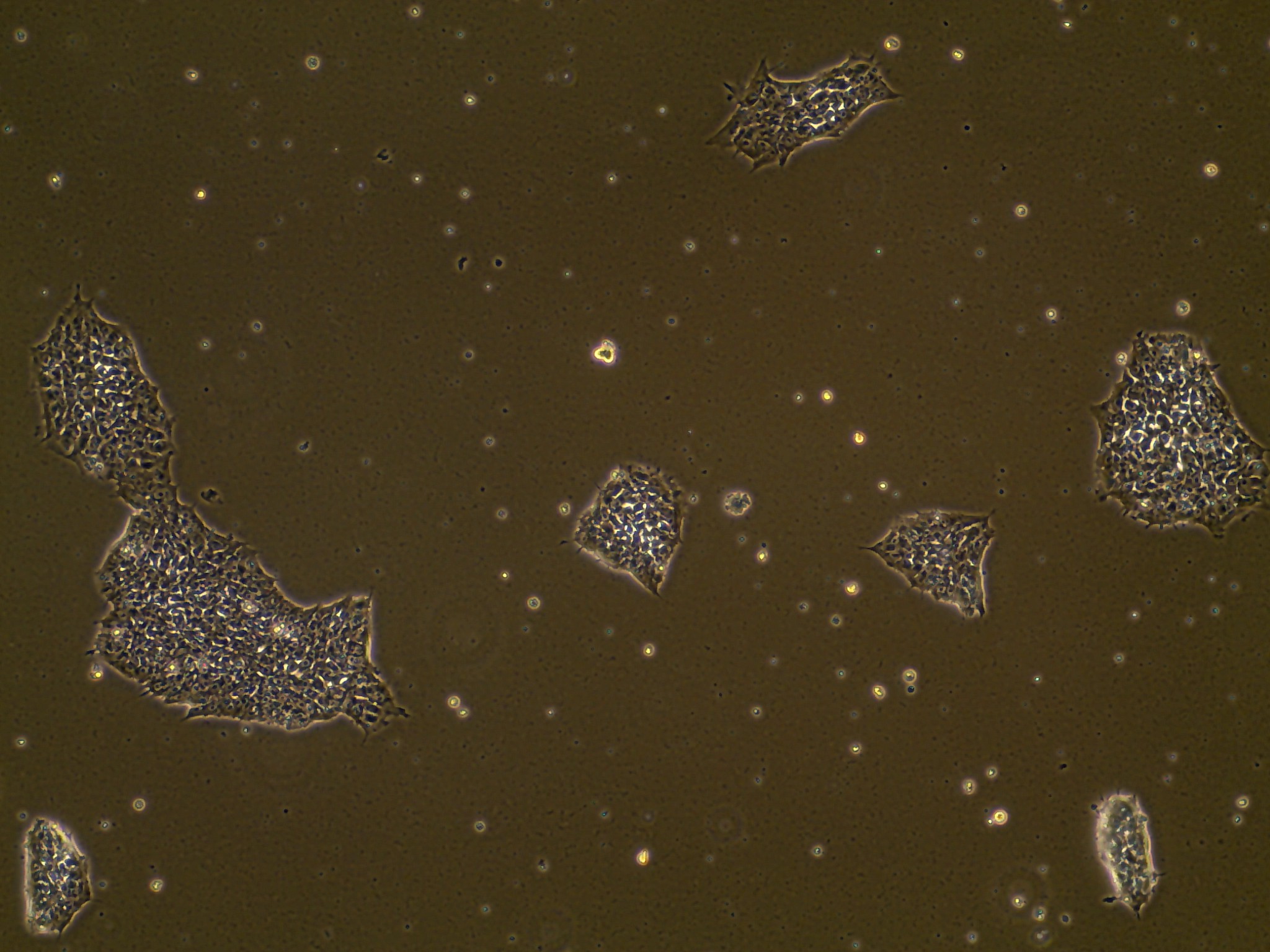
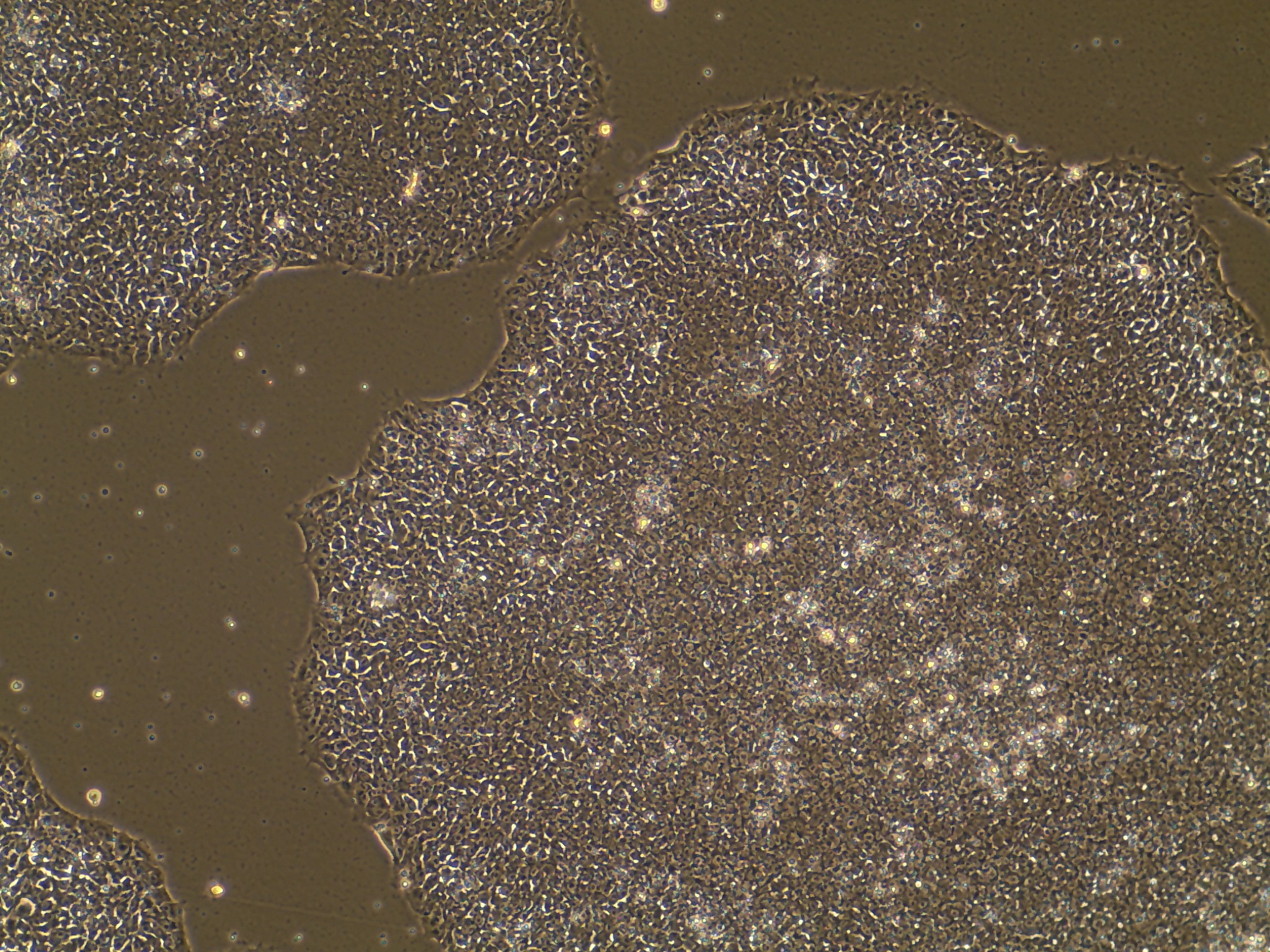
iPSC line
A CLIP contains information about a cell line including any
specific third party obligations relating to, for example,
licensing obligations or the donor consent which affect the
use of the cell line.
The EBiSC Access and Use Agreement must be completed along with an individual
Cell Line Information Pack for each line. Complete the EAUA and send to Contact@EBiSC.org
for countersignature. The EAUA must be fully signed before proceeding with your order.
A batch specific Certificate of Analysis will be available to
download once you receive your EBiSC iPSC line.
General#
Cell Line |
|
| hPSCreg name | PFIZi032-A |
| Alternative name(s) |
B214c8
|
| Cell line type | Human induced pluripotent stem cell (hiPSC) |
| Similar lines | No similar lines found. |
Provider |
|
| Depositor | Pfizer Limited - Pfizer (PFIZ) |
| Distributors |
EBiSC
|
External Databases |
|
| hPSCreg | PFIZi032-A |
| BioSamples | SAMEA104494774 |
| Cellosaurus | CVCL_VE80 |
| Wikidata | Q54947298 |
General Information |
|
| This EBiSC line can be used for: |
Yes
Research use: allowed
Clinical use: no
Commercial use: no
|
Donor Information#
General Donor Information |
|
| Sex | female |
Phenotype and Disease related information (Donor) |
|
| Diseases | A disease was diagnosed.
|
External Databases (Donor) |
|
| BioSamples | SAMEA104494775 |
hIPSC Derivation#
General |
|
| Source cell type |
A leukocyte with a single non-segmented nucleus in the mature form found in the circulatory pool of blood.
|
Reprogramming method |
|
| Vector type | Non-integrating |
| Vector | Sendai virus |
| Genes | |
| Is reprogramming vector detectable? |
No |
| Methods used |
RT-PCR
|
Vector free reprogramming |
|
Other |
|
| Derived under xeno-free conditions |
No |
| Derived under GMP? |
No |
| Available as clinical grade? |
No |
Culture Conditions#
Latest released batch |
|
| Culture medium | mTeSR™1 |
| Passage method | EDTA |
| Surface coating | Matrigel / Geltrex |
| O2 concentration | 21 |
| CO2 concentration | 5 |
| Temperature | 37 |
The following are the depositor culture conditions, they do not refer to any specific batch.
| Surface coating | Matrigel/Geltrex |
| Feeder cells |
No |
| Passage method |
Enzyme-free cell dissociation
EDTA
|
| O2 Concentration | 20 % |
| CO2 Concentration | 5 % |
| Medium |
mTeSR™ 1
|
Characterisation#
Analysis of Undifferentiated Cells
| Marker | Expressed | Immunostaining | RT-PCR | Flow Cytometry | Enzymatic Assay | Expression Profiles |
| POU5F1 (OCT-4) |
Yes |
|||||
| SSEA-1 |
Yes |
|||||
| SSEA-4 |
Yes |
|||||
| TRA 1-60 |
Yes |
Differentiation Potency
In vitro directed differentiation
In vitro directed differentiation
In vitro directed differentiation
Microbiology / Virus Screening |
|
| HIV 1 | Negative |
| HIV 2 | Negative |
| Hepatitis B | Negative |
| Hepatitis C | Negative |
| Mycoplasma | Negative |
Sterility |
|
| Inoculation for microbiological growth | No Contaminants Detected |
| Mycoplasma | Not Detected |
| Viability | Viable post-cryopreservation |
Genotyping#
Karyotyping (Cell Line) |
|
| Has the cell line karyotype been analysed? |
Yes
46, XX, modal karyotype in 20/20 cells female chromosome and complement banding pattern
Passage number: 26
|
Other Genotyping (Cell Line) |
|